Bladder cancer, or urothelial cancer, is the most common tumor of the urinary system. After surgical removal of the primary tumor, most cancers reappear within 5 years. For this reason, patients must be followed up systematically for several years after their operation. Tools for the diagnosis and monitoring of bladder cancer include cytology, which has low sensitivity, and cystoscopy, which is both invasive and expensive. Therefore, there is a significant need for biomarkers to quickly diagnose bladder cancer and its recurrence. For this purpose, non-invasive tests targeting urinary compounds would be particularly attractive.
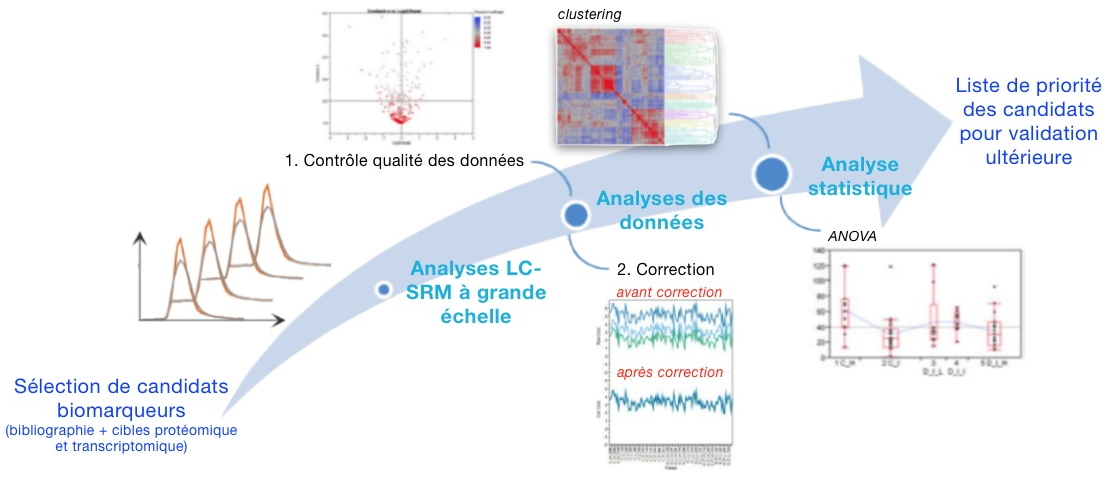
In the context of the DECanBio European project, a list of urinary biomarker candidates for bladder cancer was established using proteomics and transcriptomics screens as well as document analysis. It should be noted that, due to the low power of the statistical tests used in these so-called "high-throughput" studies, a significant proportion of these biomarker candidates consists in false discoveries. Systematic evaluation of potential biomarker candidates is the limiting step to conducting clinical tests. Such an evaluation requires the use of large-scale proteomic methods that utilize SRM (Selected Reaction Monitoring). These methods make it possible to analyze hundreds of signature peptides by evaluating thousands of signals per sample. However, due to the complexity of the analyzed samples, some signals may be interfered with by other species in the sample, which leads to inconsistencies in estimates of the abundance of some proteins.
In collaboration with the Luxembourg Public Health Research Center, researchers from our laboratory have developed an efficient and robust method for processing SRM data on a large scale. This includes reducing raw data, filtering poor quality measurements, signal normalization for each protein, and estimating protein abundance levels.
Using this method, a selection of 134 proteins potentially associated with bladder cancer was evaluated from a cohort of cancer patients and corresponding controls. This study established a priority list of the most promising biomarker candidates for the purpose of guiding future clinical validation studies.